X Chromosome Inactivation: Pioneering Research by Jeannie Lee
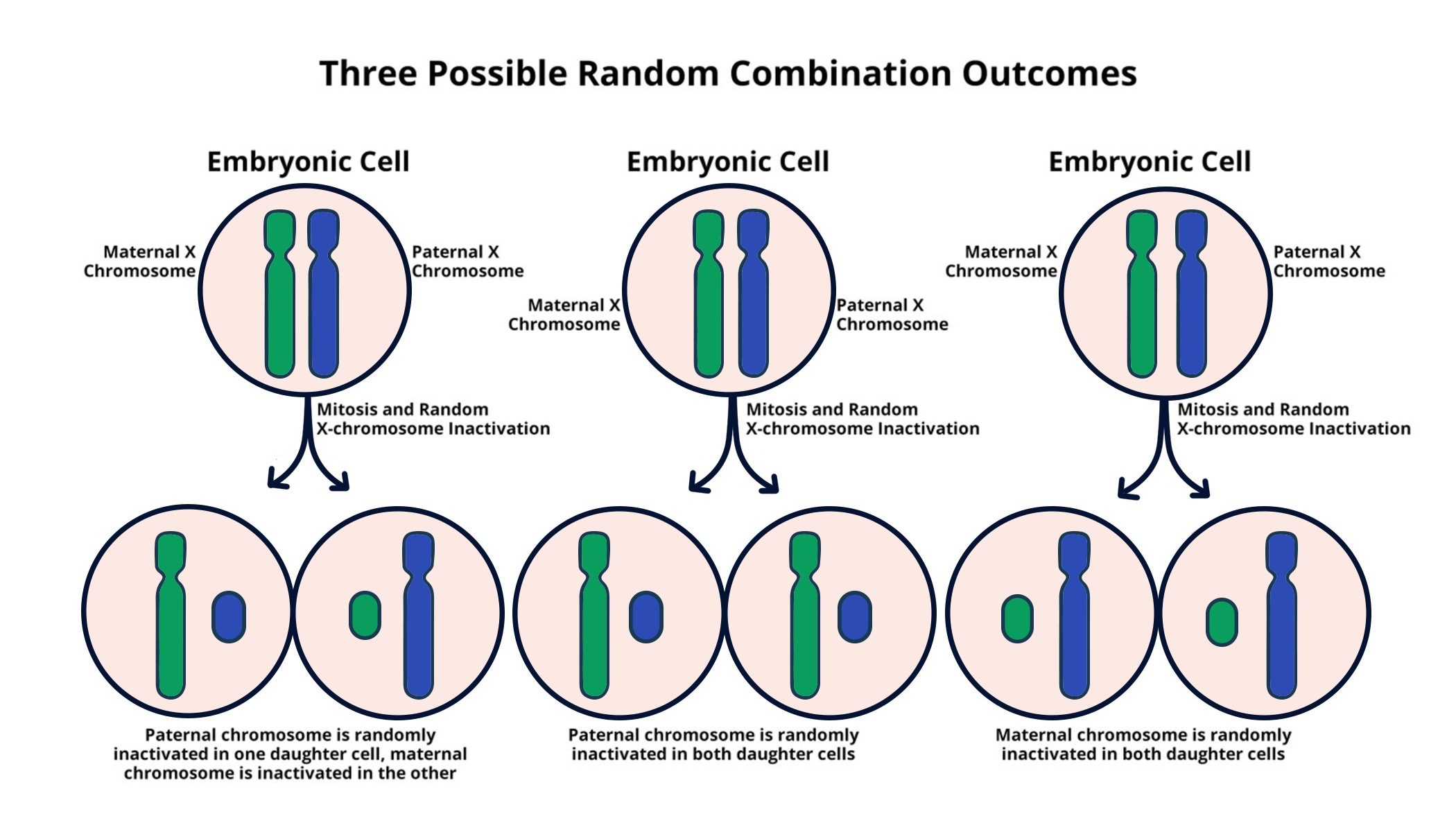
X chromosome inactivation is a remarkable cellular process that ensures females, who possess two X chromosomes, maintain genetic balance with males, who have only one. This intricate mechanism, designed to silence one of the X chromosomes, has long fascinated scientists, including Jeannie Lee and her team at Harvard Medical School. Their recent research sheds light on this chromosomal breakthrough, revealing the potential for transformative treatments for X-linked diseases such as Fragile X Syndrome and Rett Syndrome. By unraveling the complexities of how Xist RNA interacts with chromosomal structures, Lee’s work paves the way for gene therapy approaches that could alleviate the burden of these genetic disorders. As the understanding of X chromosome inactivation deepens, so too does the hope for patients grappling with the challenges posed by mutations on the X chromosome.
The process of X chromosome silencing, often referred to as dosage compensation, plays a crucial role in maintaining genetic equilibrium between the sexes. In females, who have a pair of X chromosomes, one is effectively turned off to match the single X present in males. This unique phenomenon can lead to a host of implications in understanding genetic conditions, especially X-linked diseases. Researchers like Jeannie Lee are at the forefront of exploring these mechanisms, aiming to uncover the therapeutic potential residing in unlocking the inactive X chromosome. By delving into the complexities of chromosomal functions, new avenues for treating conditions such as Fragile X and Rett syndromes are emerging, offering hope to those affected by these challenges.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a vital process in female mammals that ensures dosage compensation of X-linked genes. In females, who possess two X chromosomes, XCI silences one of these chromosomes to balance gene expression with males, who have only one X chromosome. The intricate mechanism behind this inactivation has drawn significant interest from genetic researchers, particularly in the quest to understand its implications in various x-linked diseases like Fragile X Syndrome and Rett Syndrome.
Recent advances in research led by experts like Jeannie T. Lee have shed light on the molecular intricacies of XCI. The process hinges on a unique RNA molecule, Xist, which interacts with the surrounding chromatin. The discovery of how Xist modifies the biophysical properties of the nucleoprotein matrix provides new insights into how inactivation occurs, highlighting the potential for therapeutic intervention in X-linked genetic disorders. These findings could pave the way for innovative treatment options, aimed at “unsilencing” genes on the inactivated X chromosome.
The Role of Gelatinous Substances in Gene Regulation
Research has uncovered that a gelatinous, Jell-O-like substance plays a crucial role in the regulation of X chromosome inactivation. This cytoplasm matrix not only organizes chromosomal structure but also facilitates the critical interactions needed for XCI to occur effectively. By enveloping each chromosome, this matrix prevents tangling, allowing for the precise orchestration of gene silencing mechanisms. The flexible nature of this substance makes it adaptable, enabling essential molecules like Xist to penetrate and alter the chromosomal environment.
The implications for diseases such as Fragile X Syndrome and Rett Syndrome are profound, as uncovering the relationship between chromosomal architecture and gene expression could lead to breakthroughs in treatment. If scientists can manipulate this gelatinous matrix to restore function to silenced genes, it might open new pathways for curing or alleviating the symptoms of these disorders. The ongoing research into the dynamics of this substance reflects a promising frontier in genetic therapy.
Therapeutic Potential of XCI Research
The therapeutic prospects stemming from Jeannie Lee’s investigation into X chromosome inactivation are particularly exciting for patients with X-linked diseases. By understanding how to effectively ‘unsilence’ inactivated X chromosomes, researchers aim to enable the expression of healthy genes that have been silenced due to mutations. Fragile X Syndrome, for instance, is caused by a mutation in the FMR1 gene on the X chromosome, and restoring its function could significantly improve cognitive outcomes for affected individuals.
This research aligns with ongoing efforts to develop gene therapies that target genetic mutations at their source. With promising results from initial studies, future clinical trials could test these innovative approaches, potentially leading to novel treatments that mitigate or even reverse the effects of X-linked disorders. As Lee emphasizes, the journey from understanding a basic biological process to developing transformational therapies illustrates the remarkable progress in genetic medicine.
Comparative Insights Between Male and Female Genetic Expression
Understanding X chromosome inactivation has broad implications not only for females but also for males who exhibit the effects of X-linked diseases. Although males carry only one X chromosome and do not undergo XCI, the mechanisms that silence certain genes in the presence of mutations can offer clues to developing therapeutic interventions. Fragile X Syndrome is particularly relevant here, as affected males showcase intellectual challenges stemming from the same gene affected in affected females.
By exploring the scientific nuances of gene expression in both genders, researchers can develop comprehensive treatment strategies that address the specific needs of individuals with X-linked disorders. Insights from the Lee lab indicate that while XCI is a female-specific process, understanding its principles can enhance methodologies applied to male patients as well, leading to more effective and tailored therapeutic solutions for all affected by genetic anomalies.
Jeannie Lee’s Pioneering Research Contributions
Jeannie T. Lee’s contributions to understanding X chromosome inactivation have not only propelled genetic research forward but have also illuminated pathways to potential treatments for several profound disorders. Her lab’s focus on how the Xist RNA mediates the inactivation of one X chromosome has been fundamental in unraveling the complexities of this process. This vital discovery could lead to significant advancements in curing X-linked conditions such as Fragile X Syndrome and Rett Syndrome, which affect thousands of individuals globally.
Moreover, Lee’s commitment to this area of research reflects a long-term dedication to bridging basic science with clinical applications. After years of foundational research, her recent findings represent a milestone in both genomic understanding and practical application. It is this type of breakthrough that holds the potential for changing lives and addressing unmet medical needs in genetic disorders.
Implications of X-linked Disease Research
The ongoing exploration of X chromosome inactivation and its implications for X-linked diseases signify a pivotal moment in genetic research. The challenges presented by Fragile X Syndrome and Rett Syndrome shed light on the importance of focused investigation into X-linked conditions, which often lead to significant neurological impacts and complications for those affected. By understanding the mechanisms of XCI, researchers are poised to develop interventions that could alleviate or even cure these disabling conditions.
Consequently, the integration of genetic insights and innovative therapeutic strategies is crucial for advancing treatment options. With researchers like Jeannie T. Lee spearheading efforts in this arena, the horizon for affected individuals looks brighter. It resonates with the growing push for personalized medicine, where gene therapies could one day deliver tailored solutions directly addressing the root causes of genetic disorders.
Xist and Its Role in Chromosomal Dynamics
At the heart of X chromosome inactivation lies the Xist RNA molecule, which plays a critical role in orchestrating the silencing of one of the X chromosomes in females. The interaction of Xist with chromatin results in significant changes that facilitate this process, emphasizing its importance in genetic regulation and chromosomal dynamics. The unique ability of Xist to alter the properties of the Jell-O-like matrix surrounding the chromosome demonstrates a sophisticated means of achieving gene regulation.
Understanding the specific molecular interactions and the subsequent alterations initiated by Xist not only enhances our comprehension of XCI but also opens new avenues for therapeutic interventions in X-linked diseases. Future studies could reveal how targeting Xist and manipulating its function could lead to significant breakthroughs in treating disorders like Fragile X Syndrome and Rett Syndrome, providing hope for restoring the function of genes that are crucial for neurological development.
Future Directions in X-linked Disease Treatment
The exploration of X chromosome inactivation and its implications for X-linked diseases represents a rapidly evolving field with exciting prospects for future therapies. The current research trajectory indicates a potential shift towards utilizing innovative approaches that target the silencing mechanisms of X-linked genes, specifically for conditions such as Fragile X Syndrome and Rett Syndrome. As teams led by researchers like Jeannie Lee continue to refine techniques for ‘unsilencing’ these genes, the potential for meaningful clinical applications is growing.
As we move forward, the collaboration between basic research and clinical implementation will be critical. Ongoing studies aim to establish the safety and efficacy of these new therapeutic strategies, with the hope of transitioning them into clinical trials. This dual focus on genomic understanding and practical treatment solutions will likely define the next phase of combating X-linked disorders, leading to improved outcomes and a better quality of life for affected individuals.
The Legacy of X-linked Disease Research
Research on X chromosome inactivation and its implications for X-linked diseases such as Fragile X Syndrome and Rett Syndrome has created a foundation that will inform genetic research for years to come. The insights gained from decades of study not only highlight the complexities of X-linked conditions but also underscore the significant contributions of researchers like Jeannie T. Lee. Their dedication to unraveling the mysteries of chromosomal behavior showcases an ongoing commitment to improving genetic health.
This body of work continues to inspire future generations of scientists and clinicians, as they seek to harness the potential therapeutic avenues that stem from a deeper understanding of XCI. The lessons learned throughout this research journey will undoubtedly inform the next stages of genetic therapy, leading towards innovative solutions that address the challenges presented by X-linked genetic disorders, ultimately aiming for a healthier future for affected families.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to diseases like Fragile X Syndrome?
X chromosome inactivation is a process where one of the two X chromosomes in female cells is turned off, preventing gene overexpression. This process is crucial for balancing gene dosage between males (with one X chromosome) and females (with two). In individuals with Fragile X Syndrome, mutations on the X chromosome disrupt normal function, and understanding X chromosome inactivation can lead to potential therapies that unsilence healthy genes.
How does Jeannie Lee’s research contribute to our understanding of X chromosome inactivation?
Jeannie Lee’s research has significantly advanced our knowledge of how X chromosome inactivation occurs. Her lab’s studies on the role of Xist and the gelatinous substance surrounding chromosomes have uncovered mechanisms that could unlock treatments for X-linked diseases such as Fragile X Syndrome and Rett Syndrome.
Can X chromosome inactivation be manipulated to treat Rett Syndrome?
Yes, recent advancements indicate that manipulating X chromosome inactivation may help treat Rett Syndrome. Jeannie Lee’s lab is exploring methods to unsilence the healthy genes that are silenced on the inactivated X chromosome in patients, potentially restoring normal function and alleviating symptoms.
What are the implications of chromosomal breakthroughs in X-linked diseases like Fragile X Syndrome?
Chromosomal breakthroughs, such as those uncovered by Jeannie Lee’s research on X chromosome inactivation, hold promise for therapies targeting X-linked diseases like Fragile X Syndrome. These breakthroughs may enable scientists to restore function to mutated genes by unsilencing the healthy X chromosome.
How does the ‘Jell-O-like’ substance affect X chromosome inactivation?
The ‘Jell-O-like’ substance surrounding chromosomes plays a critical role in X chromosome inactivation. It creates a flexible environment that allows the Xist RNA molecule to interact with the X chromosome, subsequently leading to its silencing and making it inactive. This process is essential for proper gene expression and could inform therapies for X-linked conditions.
Is it possible to restore gene function in individuals with Fragile X Syndrome through X chromosome inactivation research?
Research into X chromosome inactivation suggests it may be possible to restore function to genes affected by Fragile X Syndrome. By developing strategies to unsilence the inactivated X chromosome, researchers like Jeannie Lee aim to provide solutions for individuals with this and other X-linked disorders.
What potential therapies are being developed for Fragile X Syndrome based on X chromosome inactivation studies?
Potential therapies for Fragile X Syndrome being explored include methods to modify X chromosome inactivation to allow the healthy gene to be expressed. These approaches are based on findings from Jeannie Lee’s research, which focus on altering the silencing mechanisms responsible for keeping the healthy X chromosome inactive.
| Key Point | Description |
|---|---|
| X Chromosome Challenge | Females have two X chromosomes, while males have one. Inactivation of one X chromosome in females is necessary to avoid overexpression of X-linked genes. |
| Role of Xist RNA | A gene on the X chromosome produces Xist RNA, which modifies the ‘Jell-O’ substance surrounding chromosomes to facilitate X chromosome inactivation. |
| Celullar Mechanism | Xist RNA changes the material properties of surrounding ‘Jell-O’, allowing other molecules to penetrate and coat the X chromosome, thus silencing it. |
| Therapeutic Potential | Research suggests that freeing inactivated X chromosomes can potentially cure disorders like Fragile X Syndrome and Rett Syndrome by making healthy genes accessible. |
| Impact on Males | The strategies developed may also benefit males with X-linked conditions, as mutated genes can also be silenced. |
| Ongoing Research | Continued research aims to optimize treatments and explore their safety before moving to clinical trials. |
Summary
X chromosome inactivation is a vital biological process that helps balance gene expression between sexes, as females possess two X chromosomes while males only have one. This intricate mechanism, primarily mediated by the Xist RNA, allows one of the X chromosomes in females to become silenced, preventing potential gene overexpression. Recent discoveries by researchers, particularly in Jeannie T. Lee’s laboratory, indicate that manipulating this silencing process could lead to groundbreaking treatments for genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. As research progresses, the hope is to translate these findings into safe and effective clinical therapies.